Digest Journal of Nanomaterials and Biosctructures Vol. 7, No. 3, July-September 2012, p. 1205-1212
I. ANGHEL, V. GRUMEZESCUa, E. ANDRONESCUa, G. A. ANGHELb, A.M. GRUMEZESCUa*, D.E. MIHAIESCUa, M.C.CHIFIRIUCc University of Medicine Carol Davila Bucharest, Romania a Faculty of Applied Chemistry and Materials Science “Politehnica” University of Bucharest, 1-7 Polizu Street, 011061, Bucharest, Romania b ENT Clinic,Coltea Hospital, Bucharest, Romania c Faculty of Biology, University of Bucharest, Ale. Portocalelor 1-3, Bucharest, Romania
This study is aimed to evaluate a novel nanobisystem based on magnetic nanofluid and Salvia officinalis essential oil for coating the Provox voice section prostheses surfaces with desired antibiofilm properties. The essential oil microwave assisted extraction was performed in a Neo-Clevenger type apparatus and its chemical composition was settled by GC-MS analysis. Fe3O4/C18 solubilized in chloroform and oriented in magnetic field was used for coating of the catheter sections of 1 cm. The extra-shell (essential oil) was applied by adsorption in a secondary covering treatment. The fungal biofilm architecture was assessed by confocal laser scanning microscopy (CLSM). The new Fe3O4/C18/essential oil nanobiosystem exhibited a fungicidal effect and inhibited the fungal adherence, representing thus, an interesting option for the medical field, opening new directions for the design of modified surfaces with desired and controlled antibiofilm
properties.
(Received March 16, 2012; Accepted August 27, 2012)
1. Introduction
Patients who underwent laryngectomy due to larynx cancer have a possibility to regain ability to speak with the use of fluoroplastic voice prosthesis [1] (figure 1). However, the lifetime of the device is limited, and last for approximately 3–6 months, mainly due to bacterial and fungal biofilm formation, that subsequently causes deterioration of the prosthesis and malfunction of the valve mechanisms. Moreover, the biofilm can be reservoir for chronic and systemic infections [2]. Magnetite nanoparticles have attracted researchers in various fields such as medicine [3], biology [4] and materials science [5-8] due to their multifunctional properties [9], excellent biocompatibility and magnetic properties [10-12], magnetic nanoparticles are the focus of rapidly growing research efforts in controlled drug release [13,14], drug targeting [15], inhibition of microbial biofilm growth [16], resonance magnetic imaging [17,18], bone cancer treatment [3] or antimicrobial therapy [19]. Biofilms are heterogeneous communities of microorganisms entrapped in an extracellular matrix, which limits the penetration of antimicrobial drugs and antibodies [20-22]. Biofilms are characteristically composed of genetically and phenotypically diverse microbial populations [23-26] inhabiting surfaces of tissues, catheters or surgically implanted prosthetic devices: although intensively investigated the clinical the clinical relevance of fungal biofilms is often uncertain [27]. Candida albicans biofilms consist of a dense network of yeasts, hyphae and pseudohyphae encased in a selfproduced matrix of extrcellular polymeric material that shows extensive spatial heterogeneity and contributes to invasiveness [28,29]. The tendency of C. albicans to develop biofilms is clinically relevant because biofilm-associated fungal cellls are much more resistant to the traditional antifungal agents that act against their planktonic counterparts and also resist host immune factors[30,31]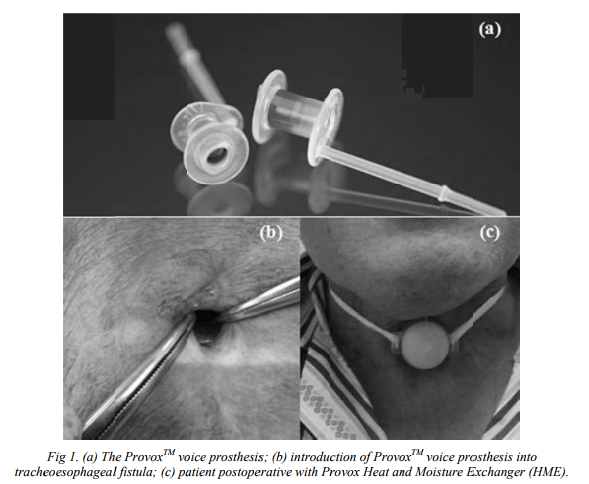
In this context, this study is focused to evaluate a novel nanobisystem based on magnetic
nanofluid and S. officinalis essential oil for coating prosthetic device surfaces with antibiofilm properties.
2. Materials and Methods
Essential oil: extraction and characterization
The essential oil microwave assisted extraction was performed in a Neo-Clevenger type apparatus and its chemical composition was settled by GC-MS analysis. GC–MS analyses were carried out on a gas chromatograph Agilent 6890 Series GC System coupled to a 5973 massselective single quadrupole detector (Agilent technologies). A DB-5MS capillary column (30 m x 0.25 mm, 0.25 mm film thickness) was used. The columba dan temperature was programmed to rise from 60 ^ C to 280 ^C at a rate of 5 ^ C/min. The carrier gas was H2 with a flow rate of 1.2 ml/min, split ratio 60:1. Operation control and the data process were carried out by Agilent Technologies ChemStation software (Santa Clara, CA, USA).
Hybrid nanobiosystem assembling procedure
Functionalized magnetite is usually prepared by wet chemical precipitation [45, 46]. Magnetic nanoparticles, Fe3O4/C18 were prepared according to our previous studies [47] and solubilized in chloroform, oriented in magnetic field and the catheter sections of 1 cm were submerged in nanofluid and extemporaneously dried because of convenient volatility of chloroform (Fe3O4/oleic acid:CHCl3 0,33%(w/v). In order to achieve core/shell/coated-shell type samples, the extra-shell (CHCl3 diluted essential oil – 160µL/mL) was applied by adsorption in a secondary covering treatment.
Fungal model
The artificial monospecific biofilms were developed by using Candida albicans 110 strain, recently isolated from wound infection and identified by using Vitek II automatic system and previously tested for susceptibility to currently used antifungals (voriconazole, itraconazole, caspofungin, amphotericin B, fluconazole, flucytosin) and some essential oils.
Biofilm development to the Provox voice section prosthesis
The microbial adherence ability was investigated in 6 multiwell plates, in which there have been placed (un)coathed prosthetic pieces of 1 cm. Plastic wells were filled with liquid medium, inoculated with 300µL 0.5 McFarland microbial suspension and incubated for 72 hours at 30°C.
After each 24 hours the culture medium was removed, the catheters were washed three times in phosphate buffered saline (PBS) in order to remove the non-adherent strains and fresh Glucose broth (GB) was added. Also viable cells counts (VCCs) have been achieved for both working
variants (coated and uncoated catheter pieces) at each 24h, in order to assess the biofilm forming ability of the tested strain. In this purpose, the fungal cells were removed from the catheter sections (by vortexing and brief sonication) and the obtained suspension was diluted in PBS, the ten fold serial dilutions being spotted in triplicates on GB and incubated at 37o C for 24 hours. After incubation, the VCCs were performed choosing the optimal dilution that allowed the development of an optmal number of microbial colonies.
Biofilm characterization
For the microbial biofilm architecture assessment, a confocal laser scanning microscopy (CLSM) was used. After 48 and 72h of incubation the samples were removed from the plastic wells, washed three times with PBS, fixed with cold methanol and dried before microscopic examination.
Samples were visualized in transmission mode by using a Leica microscope (TCS-SP CSLM model), equipped with PL FLUOTAR (40X NA0.7, electronic zoom 1) and an He-Ne laser tuned on 633 nm wavelength. A lateral resolution of about 600 nm was achieved. The Leica software was used for examining the surface topography.
3. Results and Discussion
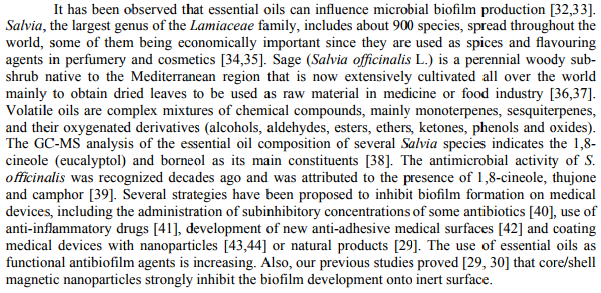
Reports on the essential oil composition of this species have been published by several authors [48,49]. Apart from some chemical variations, cis- and trans-thujones are usually the main compounds of S. officinalis essential oils [50]. The main constituents identified in the essential oils
of S. officinalis used in our experiments were cis-thujone and eucalyptol (Table 1), followed by substantial amounts of α,β-pinene and camphene. For the main constituents identified in the essential oil of S. officinalis, the obtained concentrations were similar to that obtained by other authors [51,52].
 The increasing use of indwelling medical devices in conjunction with an ageing/increasingly immunocompromised population has resulted in a surge of hospital acquired Candida spp. infections, C. albicans ranking high among nosocomial pathogens. Candida infections are frequently associated with the formation of biofilms on implantable medical devices [53]. These devices readily support biofilm formation and are responsible for a considerable percentage of clinical candidiasis cases. Several experimental parameters such as the nature of the surface material [54,55] the growth medium [56] and conditions of incubation [57] influence C. albicans biofilm formation and structure.
The increasing use of indwelling medical devices in conjunction with an ageing/increasingly immunocompromised population has resulted in a surge of hospital acquired Candida spp. infections, C. albicans ranking high among nosocomial pathogens. Candida infections are frequently associated with the formation of biofilms on implantable medical devices [53]. These devices readily support biofilm formation and are responsible for a considerable percentage of clinical candidiasis cases. Several experimental parameters such as the nature of the surface material [54,55] the growth medium [56] and conditions of incubation [57] influence C. albicans biofilm formation and structure.
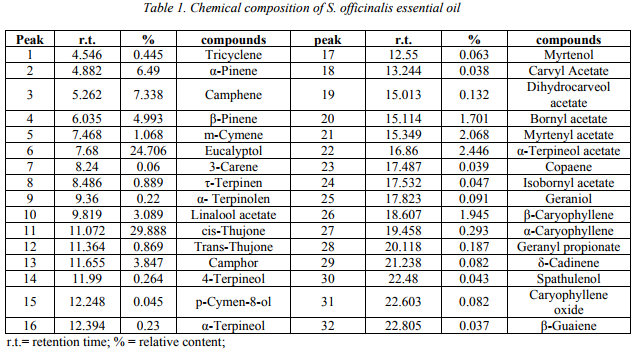
Fig 2. CLSM images of biofilms formed on the Provox voice section prosthesis (coated vs uncoated prosthetic device); a. adherent C. albicans 110 biofilm development on the surface of prosthetic device in the absence of the tested nanobyosistem at 48 h; b. the absence of C. albicans 110 yeast cells adhered to the surface of prosthetic device coated with the tested nanobyosistem at 48h; c. adherent C.
albicans 110 biofilm development on the surface of prosthetic device in the absence of the testednanobyosistem at 72 h; d. the absence of C. albicans 110 yeast cells adhered to the surface of prosthetic device with the tested nanobyosistem at 72h
The CLSM examination of the catheter sections colonized with the tested C. albicans strains showed that the tested nanobiosystem exhibited an intensive antibiofilm effect, as demonstrated by the absence of yeast cells adhered to the coated surface. In exchange, in the case of
control catheter sections with unchanged surface, the fungal cells adhered and formed aggregates, of bigger size at 72 hours, when the morphological conversion from budding yeast to a filamentous form was also more evident (Fig. 2).
The VCCs showed a decrease in the number of colony forming units (CFU) only in case of 48 hours biofilms, demonstrating that the essential oil exhibited an antifungal effect on the tested strain, reducing its multiplication rate and consequently the number of adhered cells. Conversely, at 72 hours the CFU values were quite similar with those of the control, probably due to the “consumption” of the essential oil adsorbed in the nanosystem, the increase in the multiplication rate followed by the fungal adherence to the coated surface (Fig. 3). 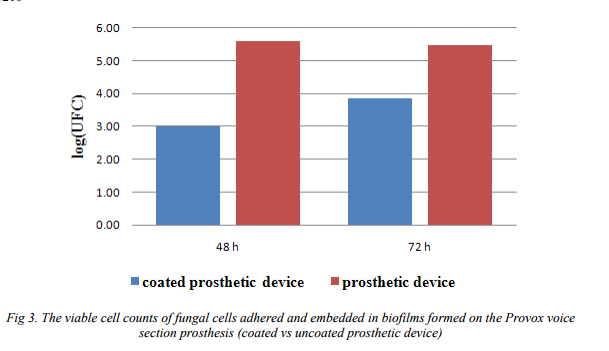
4. Conclusions
The new Fe3O4/C18/essential oil nanobiosystem exhibited a fungicidal effect and inhibited the fungal adherence, representing thus, an interesting option for the medical field, opening new directions for the design of modified surfaces with desired and controlled anti-biofilm properties. Acknowledgment
This paper is supported by the Sectoral Operational Programme Human Resources Development, financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/86/1.2/S/58146 (MASTERMAT)”.
References
[1] I. Anghel, A.G.Anghel, C.Stefan, R.Matei, Practica Medicala, 6,245 (2011).
[2] M. Nowak, P. Kurnatowski, Otolaryngologia Polska, 64, 358 (2010).
[3] E. Andronescu, M. Ficai, G. Voicu, D. Manzu, A. Ficai, J. Mat. Sci.-Mater. Med., 21 , 2237 (2010).
[4]Tian Xiao-Zhou, Wang Jian-Fang, Qian Si-Wen, Wu Wen-Jian, Gang Xu, Adv. Nat. Sci., 3, 299 (2010).
[5] D. Manzu, A. Ficai, G. Voicu B. S. Vasile, C. Guran, E. Andronescu, Mater.Plast., 47, 24 (2010).
[6] D. Ficai, A. Ficai, B. S.Vasile, M. Ficai, O. Oprea, C. Guran, E. Andronescu, Digest J. Nanomat. Biostr., 6, 943 (2011).
[7] D. Ficai, A. Ficai, M. Alexie, M. Maganu, C. Guran, E. Andronescu, Rev. Chim., 62, 622 (2011).
[8] A. M. Grumezescu, E. Andronescu, A. Ficai, C. Saviuc, D. Mihaiescu, M.C. Chifiriuc, Rom. J. Mat., 41, 383 (2011).
[9] Gui-Yin Li, Yu-ren Jiang, Ke-long Huang, Ping Ding, Jie Chenb, J. All. Comp., 466, 451 (2008).
[10] S.F. Medeiros, A.M. Santos, H. Fessi, A. Elaissari, Int. J.Pharm., 403, 139 (2011).
[11] I.H. Labouta, M. Schneider, Int. J.Pharm., 395, 236 (2010).
[12] A.M. Grumezescu, E. Andronescu, A. Ficai, D.E. Mihaiescu, B.S. Vasile, C. Bleotu, Lett. Appl. NanoBioSci., 1, 31 (2012).
[13] H. Wang, S. Wang, Z. Liao, P. Zhao, W. Su, R. Niu, J. Chang, Int. J.Pharm., 430, 343 (2011).
[14] D.E. Mihaiescu, M. Horja, I. Gheorghe, A. Ficai, A.M. Grumezescu, C. Bleotu, M.C. Chifiriuc, Lett. Appl.NanoBioSci., 1, 45 (2012).
[15] Y. Zhang, N. Kohler, M. Zhang, Biomaterials, 23, 1553 (2002).
[16] A.M. Grumezescu, C. Saviuc, A. Holban, R. Hristu, C. Croitoru, G. Stanciu, C. Chifiriuc, D. Mihaiescu, P. Balaure, V. Lazar, Biointerface Res. Appl. Chem., 1, 160 (2011).
[17]M.D. Mantle, Int. J.Pharm., 417, 173 (2011).
[18]C. Schweiger, C. Pietzonka, J. Heverhagen, T. Kissel, Int. J.Pharm., 408, 130 (2011).
[19] G. Tataru, M. Popa, J. Desbrieres, Int. J.Pharm., 404, 83 (2011).
[20] C.J. Seneviratne, L. Jin, L.P. Samaranayake, Oral Dis 14, 582 (2008).
[21] C. Saviuc, A.M. Grumezescu, M. C. Chifiriuc, C. Bleotu, G. Stanciu, R. Hristu, D. Mihaiescu, V. Lazăr, Biointerface Res. Appl. Chem., 1, 31 (2011).
[22] V.M. Bubulica, I. Anghel, A.M. Grumezescu, C. Saviuc, G.A. Anghel, M.C. Chifiriuc, I. Gheorghe, V. Lazar, A. Popescu, Farmacia., 60, 80 (2012)
[23] E. Panus, M. C. Chifiriuc, O. Banu, M. Mitache, C. Bleotu, N. Rosoiu, V. Lazăr, Biointerface Res. Appl. Chem., 1, 24 (2011).
[24] O. Banu, C. Bleotu, M. C. Chifiriuc, B. Savu, G. Stanciu, C. Antal, M. Alexandrescu, V. Lazǎr Biointerface Res. Appl. Chem., 1, 72 (2011).
[25] I. Anghel, M.C. Chifiriuc, G.A. Anghel, Farmacia, 6, 770 (2011).
[26] I. Anghel, M.C. Chifiriuc, M. Mitache , L.Marutescu , G.A. Anghel, P. Popa , P. Pelinescu, C. Bleotu, V. Lazar, Farmacia. 60, 21 (2012).
[27] A. Khodavandi, N.S. Harmal, F. Alizadeh, O. J. Scully, S. M. Sidik, F. Othman, Z. Sekawi, Kee P. Ng, P. P. Chong, Phytomedicine 19, 56 (2011).
[28] P. C. Braga, M. Culici, M. Alfieri, M. D. Sasso, International Journal of Antimicrobial Agents 31, 472 (2008).
[29] C. Saviuc, A. M. Grumezescu, E. Oprea, V. Radulescu, L. Dascalu, M. C. Chifiriuc, M. Bucur, O. Banu, V. Lazar, Biointerface Res. Appl. Chem., 1, 15 (2011).
[30] A. M. Holban, V. Lazăr, Biointerface Res. Appl. Chem., 1, 95 (2011).
[31] S.P. Hawser, L.J. Douglas, Antimicrob Agents Chemother, 39, 2128, (1995).
[32] C. Saviuc, A. M. Grumezescu, A. Holban, C. Bleotu, C. Chifiriuc, P. Balaure, V. Lazar, Biointerface Res. Appl. Chem., 1, 111 (2011).
[33] G. Ramage, S.P. Saville, B.L. Wickes, J.L. Lopez-Ribot, Appl Environ Microbiol 68, 5459 (2004).
[34] E. A. Hayouni, I. Chraief, M. Abedrabba, M. Bouix, J.Y. Leveau, H. Mohammed, M. Hamdi, International Journal of Food Microbiology 125, 242 (2008).
[35] A. P. Longaray Delamare, I. T. Moschen-Pistorello, L. Artico, L. Atti-Serafini, S. Echeverrigaray, Food Chemistry 100, 603 (2007).
[36] J. Bruneton, Pharmacognosy, Phytochemistry Medicinal Plants. Lavoisier Intercept, London, UK (1999).
[37] I. Bettaieb, N. Zakhama, W. Aidi Wannes, M.E. Kchouk, B. Marzouk, Scientia Horticulturae 120, 271 (2009).
[38] M. Z. Haznedaroglu, N. U. Karabay, U. Zeybek, Fitoterapia, 72, 829 (2001).
[39] V. Jalsenjak, S. Peljnajk, D. Kustrak, Pharmazie, 42, 419 (1987).
[40] P. Furneri, A. Garozzo, M. Musumarra, A. Scuderi, A. Russo, G. Bon- figlio, Int. J. Antimicrob. Agents 22 164 (2003).
[41] C. Arciola, L. Montanaro, R. Caramazza, V. Sassoli, D. Cavedagna, J. Biomed. Mater. Res. 42, 1 (1998).
[42] N. Cerca, S. Martins, G. B. Pier, R. Oliveira, J. Azeredo, Research in Microbiology 156, 650 (2005).
[43] C. Saviuc, A.M. Grumezescu, C.M. Chifiriuc, D.E. Mihaiescu, R. Hristu, G. Stanciu, E. Oprea, V. Radulescu, V. Lazar, Digest J. Nanomat. Biostr., 6, 1657 (2011).
[44] A. M. Grumezescu, C. Saviuc, M. C. Chifiriuc, R. Hristu, D. E. Mihaiescu, P. Balaure, G.Stanciu, V. Lazar, T. NanoBioSci., 10, 269, (2011).
[45] E. Andronescu, A.M. Grumezescu, A. Ficai, I. Gheorghe, C. Chifiriuc, D.E. Mihaiescu, V. Lazar, Biointerface Res. Appl. Chem., 2(3), 332. (2012).
[46] A.M. Grumezescu, E. Andronescu, A. Ficai, B.S. Vasile, G. Voicu, D.E. Mihaiescu, C. Bleotu, Lett. Appl. NanoBioSci., 1, 56 (2012)
[47] A.S. Buteicǎ, D.E. Mihaiescu, A.M. Grumezescu, B.Ş. Vasile, A. Popescu, O.M. Mihaiescu, R. Cristescu, Digest J. Nanomat. Biostruc., 5, 927 (2010).
[48] V. Radulescu, S. Chiliment, E. Oprea,. J. Chromatogr. A 1027, 121 (2004).
[49] D.T. Velickovic, M.S. Ristic, N.V. Randjelovic, A. Smelcerovic, J. Essent. Oil Res. 14, 453 (2002).
[50] E. Pinto , L. R. Salgueiro, C. Cavaleiro, A. Palmeira, M. J. Goncalves., Ind. Crops Prod., 26, 135 (2007).
[51] M. Marino, C. Bersani, G. Comi, International J. Food Micro., 67, 187 (2001).
[52] E. Putievsky, D. Ravid, N. Diwan-Rinzler, D. Zohary, Flav. Frag. J., 5, 121 (1990).
[53] C.A. Kumamoto, Curr. Opin. Microbiol. 5, 608 (2002).
[54] S.P. Hawser, L.J. Douglas, Infect. Immun. 62, 915 (1994).
[55] J Chandra, JD Patel, J Li, et al, Appl. Environ. Microbiol. 71, 8795 (2005).
[56] B.P. Krom, J.B. Cohen, G.E. McElhaney Feser, R.L. Cihlar, J. Microbiol. Meth. 68, 421 (2007).
[57] A.M. Gallardo-Moreno, M.L. Gonzalez-Martin, C. Perez-Giraldo, J.M. Bruque, A.C. GomezGarcia, J. Colloid. Interf. Sci., 271, 351 (2004).
