ION ANGHEL1, MARIANA CARMEN CHIFIRIUC, GEORGIANA ALINA ANGHEL
University of Medicine and Pharmacy ”Carol Davila”, Bucharest
University of Bucharest, Faculty of Biology, Microbiology Department
ENT Clinic, “Coltea” Hospital, Bucharest, Romania
corresponding authors: ionangheldoc@yahoo.com,
carmen_balotescu@yahoo.com, dr_alina.anghel@yahoo.com
Abstract
Recent studies stated that 60% to 85% of all microbial infections involve biofilms, developed on natural tissues or artificial devices which exhibited an additional resistance/tolerance to antimicrobial substances (called phenotypical or behavioural). Whereas much is known about biofilm formation on inert surfaces under laboratory conditions, little is known about mucosal biofilms.
The aim of this study was to investigate by in vitro methods the ability of microbial strains isolated from different pathologies of the rhinosinusal mucosa to adhere and develop biofilms on different substrata, in order to evaluate the contribution of this pathogenicity feature to the therapeutical difficulties encountered in the management of chronic rhinosinusitis.The study was performed on 31 randomized clinical cases of otorhinologic chronic infections, operated in the Surgical Clinic of Coltea Hospital of Bucharest, Romania. The specimens were collected intra-operatory and processed by macroscopic evaluation, microscopic examination, susceptibility testing. The isolated bacterial strains were further studied for their adherence capacity on the cellular substrate represented by HeLa cells and onto inert substrata.
In our study, the ethiological range of microbial strains isolated from rhinologicinfections was large, being represented by Gram-negative bacilli (Pseudomonas aeruginosa, Acinetobacter baumanii, Klebsiella pneumoniae, Proteus sp.), Gram-positive cocci, fungi and Actinomyces sp. strains. The majority exhibit wild antibiotic susceptibility patterns although multiresistance patterns were registered in P. aeruginosa and A. baumanii strains. All tested strains proved the ability to colonize the cellular and inert substratum. The Gram-negative nonfermentative bacilli (P. aeruginosa and A. baumanii), as well as K. pneumoniae exhibited the highest ability to colonize the inert substrate, while P. aeruginosa and the Gram-positive cocci exhibited the highest adherence indexes to the cellular substrate represented by HeLa cells. Our study demonstrated that the bacterial strains isolated from chronic rhinologic infections exhibited the ability to adhere and colonize the cellular and inert substrata. Consecutively they formed biofilms, leading to chronic, difficult to treat infections, due to the decreased susceptibility of sessile cells to antimicrobial agents compared to their planktonic counterparts.
It is therefore imperative that a standardized antimicrobial susceptibility testing protocol for such microbial strains isolated from biofilms to be implemented, from both a clinical and research standpoint.
Rezumat
Studiile de specialitate confirmă în prezent că 60% până la 85% dintre infecţiile microbiene implică biofilme dezvoltate pe ţesuturi naturale sau dispozitive artificiale. Acestea prezintă o rezistenţă suplimentară sau toleranţă la substanţe antimicrobiene (numită fenotipică sau comportamentală). La ora actuală, există foarte puţine studii despre formarea biofilmelor la nivelului mucoaselor. Scopul acestui studiu a fost de a investiga prin metode in vitro, capacitatea tulpinilor microbiene izolate din diferite patologii ale mucoasei rinosinusale de a adera şi de a dezvolta biofilme pe substrate diferite, în scopul stabilirii implicaţiilor biofilmelor microbiene în managementul rinosinuzitelor cronice. Studiul a fost realizat pe 31 de cazuri clinice randomizate, cu infecţii cronice otorhinologice, operate în cadrul Clinicii de Chirurgie a Spitalului Colţea din Bucureşti, România. Produsele patologice au fost colectate intra-operator şi analizate prin evaluare macroscopică, examen microscopic, cultivare pe diferite medii şi determinarea sensibilităţii la antibiotice. Tulpini bacteriene izolate au fost în continuare studiate pentru capacitatea lor de a adera la substratul celular reprezentat de celulele HeLa şi la substratul inert. Spectrul etiologic al tulpinilor microbiene izolate din infecţii rinologice a fost reprezentat de bacili Gram negativi (Pseudomonas aeruginosa, Acinetobacter baumanii, Klebsiella pneumoniae, Proteus sp.), coci Gram pozitivi, Actinomyces sp. şi fungi. Majoritatea tulpinilor au prezentat fenotipuri „sălbatice” de sensibilitate la antibiotice, cu excepţia tulpinilor
multirezistente de P. aeruginosa şi A. baumanii. Toate tulpinile testate au prezentat capacitatea de a coloniza substratul celular şi inert. Bacili Gram negativi non-fermentativi (P. aeruginosa şi A. baumanii), precum şi K. pneumoniae au prezentat cea mai mare capacitate de a coloniza substratul inert, în timp ce P. aeruginosa şi cocii Gram pozitivi au prezentat cele mai ridicate niveluri de aderenţă la substratul celular. Studiul de faţă demonstrează faptul că tulpinile bacteriene izolate din infecţii cronice rinologice prezintă capacitatea de a adera şi de a coloniza substraturi celulare şi inerte, precum şi de a forma biofilme. Acestea conduc la infecţii cronice, greu de tratat, datorită sensibilităţii scăzute la substanţele antimicrobiene în comparaţie cu celulele planctonice. Prin urmare, introducerea unor modele standardizate adecvate de testare asensibilităţii biofilmelor la substanţe antimicrobiene este imperativă pentru gestionarea cu succes a infecţiilor cronice rinosinusale.
Introduction
Recent research studies stated that 60% to 85% of all microbial infections involve biofilms developed on natural tissues (skin, mucosa, endothelial epithelia, teeth, bones) or artificial devices (central venous, peritoneal and urinary catheters, dental materials, cardiac valves, intrauterine contraceptive devices, contact lenses, different types of implants). In the case of biofilm development, a series of genes (40 – 60 % of the prokaryotic genome) are modulated (activated/inhibited) by complex cell-to-cell signalling mechanisms. The biofilm cells become phenotypically distinct from their counterpart – free cells, being more resistant to stress conditions (including all types of antimicrobial substances); this resistance is phenotypical, behavioural and, more recently, called TOLERANCE [1, 2]. Based on recent studies that showed the presence of biofilms in common sites of chronic infections, it has become clear that bacteria may persist on mucosal surfaces through formation of biofilms. Whereas much is known about biofilm formation on inert surfaces under laboratory conditions, little is known about mucosal biofilms [3].
Chronic rhinosinusitis (CRS) in adults is an inflammatory disease ofthe nasal and paranasal sinus mucosa defined by persistence of symptoms (nasal congestion, facial pain, headache, fever, general malaise, thick green or yellow discharge, aching teeth, rarely anosmia) for longer than 12 weeks, or by more than four episodes of rhinosinusitis occurring within year [4]. CRS affects approximately 15% of the adult population in Western industrialized countries [5].
Current therapeutic recommendations for the management of CRS rely initially on medical management with oral antibiotics and topical or oral corticosteroids. For individuals failing to respond to these therapies, endoscopic sinus surgery (ESS) is recommended for removing diseased tissue and improving drainage. However, oral antibiotic therapy is frequently unsuccessful in clearing CRS, particularly after ESS. The purpose of the present study was to investigate the implication of microbial biofilms in rhinologic chronic infections.
Materials and Methods
Bacterial strains
The study was performed on 31 randomized clinical cases ofotorhinologic chronic infections, operated in the Surgical Clinic of Coltea Hospital of Bucharest, Romania. The specimens were collected intraoperatory (purulent exudates aspirated using a sterile Pasteur pipette or a sterile syringe and ear swabs).
The study protocol was approved by the Ethical Commission of “Carol Davila” University of Medicine, Bucharest and a written informed consent was obtained from each patient.
Macroscopic evaluation
Specimens of pus, received in a syringe or in a sterile container, have been evaluated carefully for colour, consistency, and odour.
The consistency of pus varied from a turbid liquid to a very thick and sticky one. The pus was inspected for small yellow “sulfur” granules, which are colonies of the filamentous Actinomyces israelii and for the presence of small granules of different colours (white, black, red, or brown) corresponding to either filamentous bacteria or fungal mycelium. A foul feculent odour is one of the most characteristic features of an anaerobic or a mixed aerobic–anaerobic infection, although it may be lacking in some instances [6-9].
Microscopic examination
A smear for Gram-staining and examination has been made for every specimen. The smear has been examined under the oil-immersion objective (x100) and inspected carefully for the presence and the quantity (using “+” signs) of: polymorphonuclear granulocytes; Gram-positive cocci arranged in clusters, suggestive of staphylococci; Gram-positive cocci in chains, suggestive of streptococci or enterococci; Gram-negative rods resembling coliform (Escherichia coli, Klebsiella sp. etc.), other Enterobacteriaceae (Proteus sp., Serratia sp. etc.), non-fermentative rods (Pseudomonas sp.), or obligate anaerobes (Bacteroides sp.); large straight Gram-positive rods with square ends suggestive of Clostridium perfringens (the principal agent of gas gangrene), or Bacillus anthracis, the agent of anthrax; an extremely heavy and pleomorphic mixture of bacteria, including streptococci, Gram-positive and Gram-negative rods of various sizes, including fusiform rods, a picture that is suggestive of “mixed anaerobic flora”; Candida or other yeast cells, which are seen as ovoid Gram-positive budding spheres [6-9].
Culture
All specimens have been inoculated onto a blood agar plate for the isolation of staphylococci and streptococci. A MacConkey agar plate was used for the isolation of Gram-negative rods and a tube of thioglycollate broth serving as enrichment medium for both aerobes and anaerobes. The inoculated media have been incubated at 370 C for at least two days and inspected daily for growth. If staphylococci have been seen on the Gram stained smear, an additional mannitol salt agar was inoculated for obtaining pure growth and making a preliminary distinction between S. aureus and other cocci. If streptococci have been observed, their identification has been hastened by placing a differential bacitracin disc on the initial streaking area. If yeasts or fungi have been observed, the specimen has been also inoculated onto Sabouraud dextrose agar [6-9]. The well-separated colonies were carefully examined by preparing a Gram-stained smear, followed by specific identification tests. Staphylococci have been differentiated from streptococci by their morphology and by the production of catalase. The S. aureus species was confirmed phenotypically by the production of coagulase, while other coagulase-negative species have been identified by API2 Staph microgaleries. The Gram-negative aerobic bacilli have been discriminated by means of oxidase reaction, the oxidase –positive bacilli being further identified by API 20NE, while the oxidase-negative one by API 20E.
When anaerobic growth was obtained in the enrichment medium, the culture was inoculated on Brucella blood agar with hemin 5 µg/mL and vitamin K. After inoculation, the plates were incubated in anaerobic conditions for 48 hours at 37o C (anaerobic bags with reduction substances). The isolated colonies were used for macroscopic examination of colony morphology, size, pigmentation, hemolysis. Thereafter Gram-stained smears were prepared in order to establish the morphological type and Gram staining affinity, the anaerobic strains identification being performed on API 20A microtests [10]. The reading and interpretation of results were performed after 48 hours incubation at 37o C.
Susceptibility testing
Susceptibility tests were not performed on bacteria with a known sensitivity pattern, such as streptococci and Actinomyces, which are almost without exception susceptible to benzylpenicillins. For Enterobacteriaceae, non-fermentative Gram negative rods, and staphylococci the standardized disc-diffusion test (Kirby–Bauer) was used [11].
The adherence capacity of the cellular substrate represented by HeLa cells (Cravioto’s adapted method)
HeLa cells were routinely grown in minimal essential medium (MEM) enriched with 10% heat-inactivated (30 min at 56°C) fetal bovine serum (Gibco BRL), 0.1 mM non-essential amino acids (Gibco BRL), supplemented with 0.5 mL gentamycin (50 µg/mL) and incubated at 37°C for 24 h. HeLa cells monolayers were grown in free and with coverslips (coated in monolayer by Fe3O4/oleic acid core/shell tipe nanosystem) 6 multi-well plastic plates. The plates were used at 80-100% confluency. For the adherence assay, the HeLa cell monolayers were washed 3 times with phosphate buffer saline (PBS); 1 mL of bacterial suspensions of 107CFU/mL prepared in PBS was used for the inoculation of each well. The inoculated plates were incubated for 2 h at 37ºC. After incubation, the wells were washed 3 times with PBS, briefly fixed in cold ethanol (3 min), stained with Giemsa stain solution (1:20) (Merck, Darmstadt, Germany) and left to incubate for 30 min. The plates were washed, dried at room temperatureovernight, and examined microscopically with a Zeiss reversed microscope [12-14].
Development of microbial adherence onto inert substrata
The microbial biofilms were developed in 96 multi-well plates. In this purpose each well containing 200 µl of nutrient broth was inoculated with 5 µL of 0.5 McFarland microbial suspension. The plates were incubated for 24 h at 37°C. Thereafter the 96 well plates were emptied, washed 3 times with PBS, fixed with cold methanol and stained with violet crystal solution 1% for 30 min. The biofilm formed onto the plastic wells was resuspended in 30% acetic acid and the intensity of the colored suspension was assayed by measuring the absorbance at 490 nm [12-14].
Results and Discussion
The recent demonstration of the presence of biofilm in rhinosinusitis mucosa proved the implication of the biofilms in rhinosinusitis ethiopathology, directly or as mediator in physiopathology processes.
Till present, there are very few studies demonstrating biofilm morphology from mucosal samples or developed on removed frontal sinus stents from patients with chronic rhinosinusitis. The aim of this study was to investigate by in vitro methods the ability of microbial strains isolated from different pathologies of the rhinosinusal mucosa to adhere and develop biofilms on different substrata, in order to evaluate the contribution of this pathogenicity feature to the therapeutical difficulties encountered in the management of chronic rhinosinusitis.
The samples were collected from 31 patients, sex ratio 18 M/13F, aged between 35-85 years old, with chronic rhinologic infections, diagnosed using clinical and imagistic criteria (Figure 1-4).
Out of the 31 studied cases, only in 13 cases there were isolated bacterial and fungal strains (2 anaerobic and 11 aerobic strains). Out of the 18that were negative (without any microbial growth both in aerobic and anaerobic conditions), 10 cases were submitted to pre-operatory empiric therapy at the moment of specimen collection. The absence of microbial growth may be explained by the efficiency of antibiotic therapy but at the same time by other aspects related to the pathological substrate of the morbid process, as failure of specimen processing with special reference to anaerobic strains [10].
Cultures of individuals with chronic chronic rhinologic infections showed higher than usual incidences of Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae and Haemophilus influenza persistance. On sensitivity testing, these usually are susceptible to available oral agents. However, oral antibiotic therapy frequently is unsuccessful in clearing the infection. The failure of antibiotic therapy is somewhat surprising, given the high success rate of antibiotics in the treatment of acute bacterial maxillary rhinosinusitis. The presence of bacterial biofilms may help explain the lack of effectiveness of oral antibiotics.
In our study, the etiology was led by the Gram-negative bacilli (2) Pseudomonas aeruginosa, 1 Acinetobacter baumanii, 1 Klebsiella pneumoniae, 1 Proteus sp.) (Figure 5), followed by the Gram-positive cocci (1 S. aureus, 1 S. sciuri, 1 Str. pyogenes, 1 Enterococccus faecium/faecalis) (Figure 6-8) and fungi (Candida albicans, Aspergillus flavus). Concerning the anaerobic strains, only two Actinomyces strains were recovered from the analyzed samples (Figure 9).
Concerning the susceptibility to antibiotics, the Klebsiella pneumoniae strain was resistant to ampicillin, first generation cephalosporins and amoxicillin + clavulanic acid, while Proteus exhibited resistance to amoxicillin + clavulanic acid, chloramphenicol and tetracycline. One P. aeruginosa strain proved to be susceptible to all tested antibiotics, while the other one, as well as the Acinetobacter strain, proved to be multiresistant to the majority of the tested antibiotics, excepting some aminoglycosides and imipenem. The Gram-positive cocci also proved to be susceptible to all tested antibiotics.
Concerning the fungal strains, Candida albicans proved to be susceptible to all tested antimicrobial agents, while Aspergillus flavus was resistant to ketoconazole, amphotericin B, clotrimazole and miconazole. By virtue of their nature, bacteria in biofilms are much less susceptible to antibiotics. Minimally inhibitory concentrations (MIC) effective on an organism in planktonic form are ineffective against the same organism when present in the biofilm form. The treatment should be based on antibiotic doses which are efficient on bacteria included in biofilms [15].
In some usually sterile tissue or organs, biofilms are monospecific, whereas when they developed on some exposed tissue (rhinosinusal mucosa, farinx, gut) biofilms are mixt and hard to treat. Chronic rhinologic infections appear to be a polymicrobial infection with the potential for involvement with a diverse range of bacteria. The study of bacterial biofilm on human tissue and medical devices, their involvement in chronical infections and their resistance to actual antibiotic therapy represent new directions for research in the future [16].
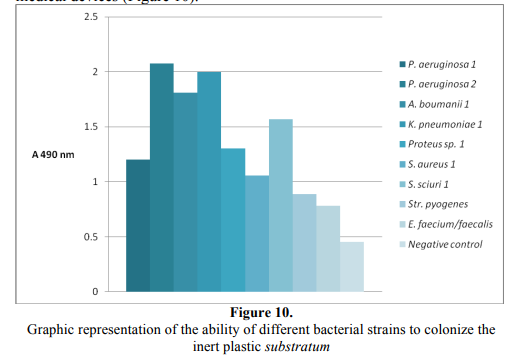
All tested strains proved the ability to colonize the cellular and inert substrate. Among the aerobic bacterial strains, the Gram-negative nonfermentative bacilli (P. aeruginosa and A. boumanii), as well as K. pneumoniae exhibited the highest ability to colonize the inert substrate, quantified by slime production, explaining their ability to survive in the hospital environment and to become nosocomial agents, often implicated in biofilms’ associated infections, following the colonization of different medical devices (Figure 10).
Concerning the ability of the tested strains to colonize the cellular substrate, three distinct patterns of adherence have been investigated during this study: localized adherence (LA), in which bacteria attach to and form microcolonies in distinct regions of the surface; diffuse adherence (DA), in which bacteria adhere evenly to the whole cell surface, and aggregative adherence (AggA), in which aggregated bacteria attach to the cell in a stacked-brick arrangement.
The adherence index was expressed as the ratio between the numbers of the eukaryotic cells with adhered bacteria: 100 eukaryotic cells counted on the microscopic field [17-19] (Figure 11).
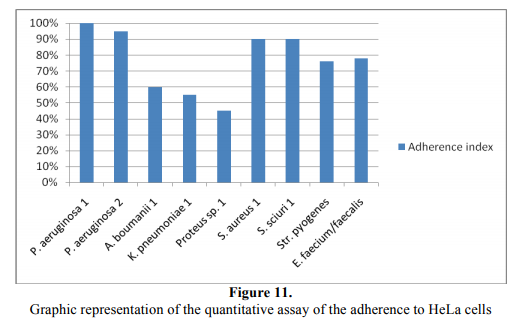
P. aeruginosa and the Gram-positive cocci exhibited the highest adherence indexes, as compared with the rest of the tested strains. In the case of A. boumanii and K. pneumoniae strains the lower capacity to adhere to the eukariotic cells could be explained by the presence of a polysaccharide capsule in these strains, negatively charged. Such is the eukariotic cell membrane, opposing to the intimate interaction between bacterial adhesins and complementary eukariotic cell receptors. The most frequent adherence pattern was the mixed localized –aggregative one.
Conclusions
In our study, the etiological range of microbial strains isolated from rhinologic infections was large, being represented by Gram-negative bacilli (P. aeruginosa, A. baumanii, K. pneumoniae, Proteus sp.), Gram-positive cocci, fungi and Actinomyces strains, the majority exhibiting wild antibiotic susceptibility patterns although multiresistance patterns were registered in P. aeruginosa and A. baumanii strains. All the tested strains proved the ability to colonize the cellular and inert substrate. The Gram-negative non fermentative bacilli (P. aeruginosa and A. baumanii), as well as K. pneumoniae exhibited the highest ability to colonize the inert substrata, while P. aeruginosa and the Gram-positive cocci exhibited the highest adherence indexes to the cellular substrate represented by HeLa cells. Our study demonstrated that the bacterial strains isolated from chronic rhinologic infections exhibited the ability to adhere and colonize the cellularand inert substrata. Consecutively they form biofilms, leading to chronic, difficult to treat infections, due to the decreased susceptibility of sessile cells to antimicrobial agents compared to that of planktonic cells. It is therefore imperative that a standardized antimicrobial susceptibility testing protocol for such microbial strains isolated from biofilms be implemented, from both a clinical and research standpoint.
Aknowledgements
The results presented in this paper have been obtained with the financial support obtained from the Human Resources Project no. 135/2010 (Contract no. 76/2010).
References
1. Veronica Lazãr, Mariana Carmen Chifiriuc. Architecture and physiology of microbial biofilms, Roum. Arch. Microbiol. Immunol., 2010, 69 (3) 92-98
2. Veronica Lazãr, Mariana Carmen Chifiriuc. Medical significance and new therapeutical strategies for biofilm associated infections, Roum. Arch. Microbiol. Immunol., 2010, 69 (3) 121-127
3. Crina Saviuc, Alexandru Mihai Grumezescu, Eliza Oprea, Valeria Radulescu, Luminita Dascalu, Mariana Carmen Chifiriuc, Marcela Bucur, Otilia Banu, Veronica Lazar: Antifungal activity of some vegetal extracts on Candida biofilms developed on inert substratum, Biointerface Research in Applied Chemistry, 1, 1, 2011, 015-023
4. Ion Anghel, Ion Grecu,. Orbito-oculare complications with rhinosinusal ethiology, Otorinolaringologia, 1999, 20;1-2:14-16.
5. R.J. Harvey, V.J.Lund. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research, Rhinology, 2007; 45:1:3-14;
6. ***WHO, Basic laboratory procedures in Clinical bacteriology, 2nd Ed, 2003.
7. B.T. Hopkins. Laboratory Notes. Guide to Laboratory and Diagnostic tests, 2005.
8. REMIC. Reférentiel en Microbiologie médicale, Société Française de Microbiologie, Collection Vivactis plus éditions, 2008.
9. G.F.,Brooks, K.C. Caroll, J.S. Butel, S.A. Morse eds. Jawetz, Melnick, & Adelberg’s Medical Microbiology, 24th Edition.
10. Anca –Michaela Israil, Cristina Delcaru, R.S. Palade, Carmen Chifiriuc, Carmen Iordache, D.Vasile, M.Grigoriu, D.Voiculescu. Bacteriological Aspects Implicated in Abdominal Surgical Emergencies, 2010, Chirurgia,6, 2010 105: 779-787
11. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Tests; Eighteen informational supplement M100-S18, 2008
12. Otilia Banu, Coralia Bleotu, Mariana Carmen Chifiriuc, Bogdan Savu, George Stanciu, Cristina Antal, Magdolna Alexandrescu, Veronica Lazǎr: Virulence factors of Staphylococcus aureus and Pseudomonas aeruginosa strains involved in the etiology of cardiovascular infections, Biointerface Ress. in App. Chem., 1, 2, 2011, 72-77
13. Crina Saviuc, Alexandru Mihai Grumezescu, Alina Holban, Coralia Bleotu, Carmen Chifiriuc, Paul Balaure, Veronica Lazar, Phenotypical studies of raw and nanosystem embedded Eugenia carryophyllata buds essential oil antibacterial activity on Pseudomonas aeruginosa and Staphylococcus aureus strains, Biointerface Ress. in App. Chem., 1, 3, 2011, 111-118, 2011
14. Emilia Panus, Carmen-Mariana Chifiriuc, Otilia Banu, Magda Mitache, Coralia Bleotu, Natalia Rosoiu, Veronica Lazar: Comparative study of resistance and virulence markers