ION ANGHEL, ALEXANDRU MIHAI GRUMEZESCU, ALINAGEORGIANA ANGHEL, IULIAN CHIREAC4, LUMINITA MARUTESCU4, DAN EDUARD MIHAIESCU2, MARIANA CARMEN CHIFIRIUC University of Medicine Carol Davila Bucharest, Romania
Politehnica University of Bucharest, Faculty of Applied Chemistry and Materials Science, Bucharest, Romania
ENT (Otorhinolaryngology) Clinic, Coltea Hospital, Bucharest, Romania
University of Bucharest, Faculty of Biology, Microbiology Immunology Department, Bucharest, Romania
corresponding author: ionangheldoc@yahoo.com
Abstract
An adapted difussimetric method was used in order to assess the potentiator effect of some natural (NZ) and synthetic zeolites (SZ)with well defined nanopores on the antibiotic (ATB) susceptibility of Enterococcus fecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Escherichia coli strains to some of the currently used antibiotics, i.e. neomycin, polymixin, norfloxacin and cefotaxime. Our results show that the natural and synthetic zeolites with well defined nanopores release antibiotics with different chemical structures in an active form and improve their antimicrobial effect against Gram-positive and Gram-negative bacterial strains, constituting promissing delivery systems for various classes of commonly used antibiotics.
Rezumat
O metodă difuzimetrică adaptată a fost utilizată în vederea testării efectului potenţiator al unor zeoliţi naturali (NZ) şi sintetici (SZ) cu nanopori bine definiţi asupra antibioticelor (ATB) susceptibile la tulpinile de Enterococcus fecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis şi Escherichia coli la unele antibioticeutilizate în prezent, de ex. neomicina, polimixina, norfloxacin şi cefotaxim. Rezultatele arată că zeolitul natural şi sintetic cu nanopori bine definiţi eliberează antibioticele cu structuri chimice diferite într-o formă activă şi îmbunătăţirea efectului antimicrobian asupra tulpinilor bacteriene Gram-pozitive şi Gram-negative, constituind sisteme promiţătoare de administrare pentru diferite clase de antibiotice utilizate în mod obişnuit.
Introduction
Hybrid materials are new materials with improved properties withapplications in medicine [1], water purification [2] or drug delivery [3,4].
The development of materials with well-arranged structures [5,6] offers new possibilities for incorporating biological agents for controlling the kineticsof their release from the matrix [7,8,9]. One of the interesting features of ordered hybrid solids for controlled drug release is the multitude of possible modifications that can be used to both adjust surface functionality and change textural properties [10,11].
At least 17 different classes of antibiotics have been produced to date. Unfortunately, for each one of these classes at least one mechanism of resistance (and many times more than one) has been developed over the years by bacterial cells [12]. The continuing rise in microbial drug resistancehas led to widespread problems in the treatment of bacterial infections [13].
The emergence of bacterial resistance to the existent antibacterial drugs is impairing not only the therapy, but also favor bacteria to remain alive and viable in the hospital environment, thus affecting vulnerable patients who are at a higher risk and in whom eventually they cause serious nosocomial infections [14,15,16].
Incorporation of drugs into inorganic carriers with the purpose of delivering the active compound at a controlled rate is a major issue of interest for both materials science and medicine science [17]. The ability to fully characterize drug systems, to provide drug targeting with high specificity and drug delivery with integrated controlled release are all major challenges to the pharmaceutical industry [18]. Silica has been investigated for applications in biomedical devices [19], due to its low toxicity and biocompatibility, and to its hydrophilic character and porous structure [20] that can be tailored to control the diffusion rate of an adsorbed or encapsulated drug [21]. Mesoporous materials posses a network of channels and voids of well-defined size in the nanoscale range (2–50 nm) [22]. Zeolites have a well-defined nanopore structure with a crystalline framework. Zeolites can be used to load agents as well as to release them, and the high ion-exchange capacity improves the loading of agents [23]. This particular pore architecture makes them suitable candidates for hosting and further delivering under appropriate conditions of a variety of molecules of pharmaceutical interest [24].
Starting from the natural and synthetic zeolites, in the present contribution the study is extended to evaluate the potentiator effect ofzeolites on antibiotics activity. The major advantage is that the introduction of drugs onto the zeolites effectively delays the drug release process, improves the antibiotic effect and simultaneously, retains the high drug loading capacity of mesoporous silica.
Materials and Methods
Antibiotic entrapping onto zeolites nanopores
Commercially available ZSM-5 (synthetic zeolite – SZ) and permutit (natural zeolite – NZ) were used to prepare hybrid materials through absorption of the antibiotics onto nanopores. The amount of the antibiotic adsorbed on the zeolites support was 3%. The zeolite and the respective antibiotic (neomycin, polymixin, norfloxacin and cefotaxime) to be adsorbed were introduced in a grinding mortar. The mix was ground with 2mL of ultrapure water until the latter completely evaporated at 40^ C.
FT-IR
A Nicolet 6700 FT-IR spectrometer (Thermo Nicolet, Madison, WI) connected to the software of the OMNIC operating system (Version 7.0 Thermo Nicolet) was used to obtain the FT-IR spectra of hybrid materials. The samples were placed in contact with attenuated total reflectance (ATR) on a multibounce plate of ZnSe crystal at controlled ambient temperature (25o
C). FT-IR spectra were collected in the frequency range of 4,000–650 cm-1 by co-adding 32 scans and at a resolution of 4 cm-1 with strong apodization. All spectra were rationed against a background of an air spectrum. After every scan, a new reference air background spectrum was taken. The plate was carefully cleaned by wiping with hexane twice followed by acetone and dried with soft tissue before filling in with the next sample. The spectra were recorded as absorbance values at each data point in triplicate.
Scanning Electron Microscopy (SEM)
The NZ and SZ were assessed by SEM analysis performed on a HITACHI S2600N electron microscope, at 19 and 25 kV, in primary electrons fascicle, on samples covered with a thin silver layer.
The antibiotic potentiator effect of the NZ and SZ
An adapted diffusion method was used in order to assess the potentiator effect of natural and synthetic zeolites on the antibiotic susceptibility of the tested E. fecalis, Ps. aeruginosa, S. aureus, B. subtilis and E.coli strains to some of the currently used antibiotics, chosen according to CLSI recomandations i.e. neomycin, polymixin, norfloxacin and cefotaxime. Qualitative screening of the susceptibility of different microbial strains to NZ/ATB and SZ/ATB has been accomplished through an adapted diffusion method, on Mueller Hinton solid medium previously seeded with a bacterial inoculum adjusted to a density correponding to 0.5 McFarland standard [29,30,31]. In this purpose, 5 µL from a stock solution of the tested product, containing 30 µg of antibiotic, as well as the antibiotic control used at the same concentration, were distributed in spots on the Petri plates. The results’ reading was performed by measuring the bacterial growth inhibition zones’ diameters around the spots. The used solvent, dimethyl sulfoxide (DMSO) [32,33], was comparatively tested for its potential antimicrobial activity. The plates were incubated 24h at 37°C, and the differences between inhibition zones diameters were quantified [34,35].
Results and Discussion
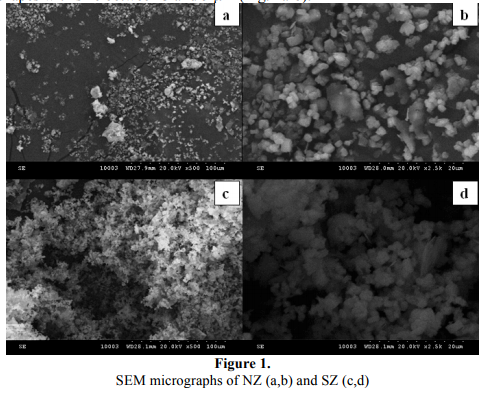
The shape and size of SZ and NZ microparticles were studied by SEM. Fig. 1 shows SEM images of the two zeolites. It can be seen that the as-received SZ sample is under the form of nearly spherical aggregates of ca. 2 µm in average sizes (Fig. 1c-d), while NZ sample exhibit almost cubic shapes with size between 3 and 5 µm (Fig. 1a-b).
 FT-IR spectroscopic data provide useful information about the zeolite/ATB relationship. The stability of the drugs used in this experiment was proved with FT-IR spectra plotted in figure 2-6 and also by the microbiological assay, demonstrating that the antibiotic was released in an active form. Based on these arguments, we have concluded that our absorption processes were performed successfully without changing the structure of antibiotics. The changes in area of the bands and many peaks in the “fingerprint” region were observed. The “fingerprint” region of the ATB/NZ and ATB/SZ spectra shows clear differences after loading the drugs.
FT-IR spectroscopic data provide useful information about the zeolite/ATB relationship. The stability of the drugs used in this experiment was proved with FT-IR spectra plotted in figure 2-6 and also by the microbiological assay, demonstrating that the antibiotic was released in an active form. Based on these arguments, we have concluded that our absorption processes were performed successfully without changing the structure of antibiotics. The changes in area of the bands and many peaks in the “fingerprint” region were observed. The “fingerprint” region of the ATB/NZ and ATB/SZ spectra shows clear differences after loading the drugs.
FT-IR spectra of norfloxacin loaded into NZ and SZ nanopores
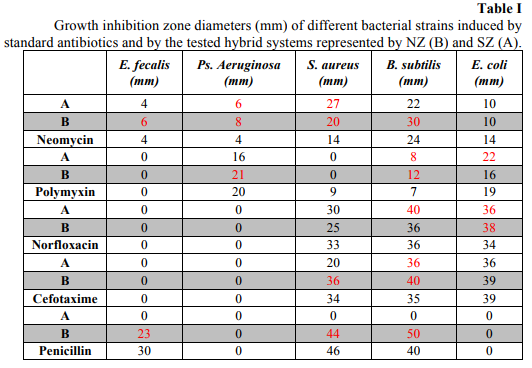
The hybrid antibiotic-zeolite systems exhibited different antimicrobial activities as compared to the standard antibiotics, tested at the same concentration. The NZ (natural zeolite) improved the activity of neomycin for the majority of the tested bacterial strains, excepting E. coli, of polymixin in the case of E. coli, of norfloxacin and cefotaxime against S. aureus and B. subtilis and of penicillin in the case of E. faecalis (Table I). The synthetic zeolite (SZ) improved the antimicrobial activity of neomycin on Ps. aeruginosa and S. aureus, of polymixin and norfloxacin on B. subtilis and E. coli and of cefotaxime against B. subtilis.
B. subtilis proved to be by far the most susceptible to the tested hybrid systems, showing increased susceptibility to at least one of the two tested combinations of all tested antibiotics, and the incorporation in the natural zeolite proved to be more efficient in the release of the antibiotics in active forms and improvement of their antimicrobial activities
 Conclusions
Conclusions
Our results show that the natural and synthetic zeolites with well defined nanopores release antibiotics with different chemical structures in an active form and improve their antimicrobial effect against Gram-positive and Gram-negative bacterial strains, constituting promissing delivery systems for various classes of commonly used antibiotics.
Acknowledgments
The financial support of the European Commission through European Regional Development Fund and of the Romanian state budget, project POSCCEO2.1.2-2009-2, ID 691, “NEW MESOPOROUS ALUMINOSILICATE MATERIALS FOR CONTROLLED RELEASE OF BIOLOGICALLY-ACTIVE SUBSTANCES”, Human Resources Project no. 135/2010 (Contract no. 76/2010) and Ideas Project no. 154/2011 are gratefully acknowledged.
References
1. Andronescu E, Ficai M, Voicu G, Manzu D, Ficai A: Synthesis and characterization of collagen/ hydroxyapatite – magnetite composite material for bone cancer treatment. J. Mat. Sci.-Mater. Med. 2010, 21(7): 2237-2242.
2. Ficai D, Ficai A, Alexie M, Maganu M, Guran C, Andronescu E: Amino-functionalized Fe3O4/SiO2/APTMS nanoparticles with core-shell structure as potential materials for heavy metals removal. Rev. Chim. (Bucharest) 2011, 62:622-625.
3. Grumezescu AM, Andronescu E, Ficai A, Saviuc C, Mihaiescu D, Chifiriuc MC: DeaeCellulose/Fe3O4/Cephalosporins hybrid materials for targeted drug delivery. Rom. J. Mat. 2011, 41(4):383-387.
4. Ha JU, Xanthos M: Drug release characteristics from nanoclay hybrids and their dispersions in organic polymers. Int. J. Pharm. 2011, 414(1-2):321-331.
5. Voicu G, Ghitulica CD, Dinu E, Andronescu E: In vitro behavior of dicalcium silicate obtained through the sol-gel method. Rom. J. Mat. 2011, 41(3):229-233.
6. Rahmat D, Sakloetsakun D, Shahnaz G, Perera G, Kaindl R, Bernkop-Schnürch A: Design and synthesis of a novel cationic thiolated polymer. Int. J. Pharm. 2011, 411(1-2):10-17.
7. Grumezescu AM, Saviuc C, Holban A, Hristu R, Croitoru C, Stanciu G, Chifiriuc C, Mihaiescu D, Balaure P, Lazar V: Magnetic chitosan for drug targeting and in vitro drug delivery response. Biointerface Res. Appl. Chem. 2011, 1(5):160-165.
8. Mihaiescu DE, Grumezescu AM, Balaure PC, Mogosanu DE, Vanessa Traistaru, Magnetic scaffold for drug targeting: evaluation of cephalosporins controlled release profile, Biointerface Res. Appl. Chem. 2011, 1(5):191-195.
9. Planinšek O, Kovačič B, Vrečer F: Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int. J. Pharm. 2011, 406(1-2):41-48.
10. Grumezescu AM, Mihaiescu DE, Tamaş D: Hybrid materials for drug delivery of rifampicin: evaluation of release profile. Biointerface Res. Appl. Chem. 2011, 1(6):229-235.
11. Shen SC, Ng WK, Chia L, Hu J, Tan RBH: Physical state and dissolution of ibuprofen formulated by co-spray drying with mesoporous silica: Effect of pore and particle size. Int. J.Pharm. 2011, 410(1-2)188-195.
12. Alanis AJ: Resistance to Antibiotics: Are We in the Post-Antibiotic Era?. Arch. Med. Res. 2005, 36:697–705.
13. Turos E, Reddy GSK, Greenhalgh K, Ramaraju P, Abeylath SC, Jang S, Dickey S, Lim DV: Penicillin-bound polyacrylate nanoparticles: Restoring the activity of b-lactam antibiotics against MRSA. Bioorg. Med. Chem. Lett. 2007, 17:3468–3472.
14. Kollef MH, Fraser VJ: Antibiotic resistance in the intensive care unit. Ann. Intern. Med. 2001;134:298–31
15. Nser S, Di Pompeo C, Soubrier S, Delour P, Lenci H, RousselDelvallez M, Onimus T, Saulnier F, Mathieu D, Durocher A. Firstgeneration fluoroquinolone use and subsequent emergence of multiple drug-resistant bacteria in the intensive care unit. Crit. Care. Med. 2005, 33:283–328.
16. Sefton AM: Mechanisms of antimicrobial resistance. Drugs. 2002, 62: 557–566.
17. Horcajada P, Márquez-Alvarez C, Rámila A, Pérez-Pariente J, Vallet-Reg M: Controlled release of Ibuprofen from dealuminated faujasites. Solid State Sci. 2006,8:1459–1465.
18. Tsz Y, Turner A, Roberts CJ, Davies MC: Scanning probe microscopy in the field of drug delivery. Adv. Drug Deliv. 19. Dhanasingh S., Mallesha J, Hiriyannaiah J: Preparation, characterization and antimicrobial studies of chitosan/silica hybrid polymer. Biointerface Res. Appl. Chem. 2011, 1(2):048- 056.
20. Comanescu C, Palade P, Ficai D, Guran C: New mesoporous materials based on functionalized SBA-15 with 3-aminopropyl tri(m)ethoxysilane. Rom. J. Mat. 2011, 41(2):141-146.
21. Son SJ, Bai X, Nan A, Ghandehari H, Lee SB: Template synthesis of multifunctional nanotubes for controlled release. J. Control. Release. 2006, 114:143–152.
22. Horcajada P, Ramila A, Perez-Pariente J, Vallet-Reg M: Influence of pore size of MCM-41 matrices on drug delivery rate. Microp. Mesop. Mat. 2004, 68:105–109.
23. Payra P, Dutta PK: Handbook of Zeolite Science and Technology, Dekker, New York, 2004, 1–19.
24. Fisher KA, Huddersman KD, Taylor MJ: Comparison of Micro- and Mesoporous Inorganic Materials in the Uptake and R elease of the Drug Model Fluorescein and Its Analogues. Chem. Eur. J. 2003, 9:5873–5878.
25. Saviuc C, Grumezescu AM, Holban A, Chifiriuc C, Mihaiescu D, Lazar V: Hybrid nanostructurated material for biomedical applications. Biointerface Res. Appl. Chem. 2011, 1(2):64-71.
26. Saviuc C, Grumezescu AM, Holban A, Bleotu C, Chifiriuc C, Balaure P, Lazar V: Phenotypical studies of raw andnanosystem embedded Eugenia carryophyllata buds essential oil antibacterial activity on Pseudomonas aeruginosa and Staphylococcus aureus strains. Biointerface Res. Appl. Chem., 2011, 1(3):111-118.
27. Anghel I, Chifiriuc MC, Anghel GA: Pathogenic features and therapeutical implications of biofilm development ability in microbial strains isolated from rhinologic chronic infections. Farmacia. 2011, 59,(6):770-783.
28. Saviuc C, Grumezescu AM, Oprea E, Radulescu V, Dascalu L, Chifiriuc MC, Bucur M, Banu O, Lazar V: Antifungal activity of some vegetal extracts on Candida biofilms developed on inert substratum. Biointerface Res. Appl. Chem., 2011, 1(1):15-23.